Remember Remember, the Fifth of November…
This evening is Guy Fawkes Night and soon revelers across the country will be ooh’ing and aah’ing as the sky is lit up with an array of beautiful colours (and in the case of Glasgow, some interesting shapes – this is a family-friendly blog, google it!).

The ill-fated Guy Fawkes, mastermind of the failed ‘Gunpowder Plot’ to blow up the Houses of Parliament (photo from WIkipedia).
But what is it that gives fireworks their colour? The answer of course lies in chemistry…
Fireworks contain metal compounds called metal salts and metal oxides. When these are heated, the atoms of the metal absorb energy, causing electrons to become ‘excited’ and move to a higher energy level. As the heat dissipates, the electrons plummet back down to their lower energy level and excess energy is released as light.
The atoms of different metals emit different amounts of energy, which produces different wavelengths of light (i.e. different colours).
For example, when the metal salt sodium nitrate is heated, sodium atoms absorb energy and are excited; when the electrons fall back to their lower energy state, energy is released as light in the wavelength of about 500-600nm, which is the wavelength of yellow-coloured light (see below).
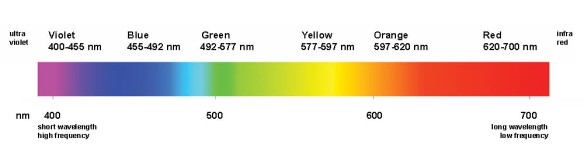
Visible light spectrum. Short wavelengths of visible light produce violet and blue colours; longer wavelengths emit orange and red colours.
Here are some metals commonly found in fireworks and their associated pretty colours….

Copper (photos from Wikipedia, http://m-imajo.main.jp)
Purple fireworks can be produced using a mixture of strontium and copper compounds
So there we have it. Now you’re armed with some quality science to dazzle your pals with as you brave the crowds and rogue sparklers at Glasgow Green tonight:
“What’s your favourite firework? I like the the Catherine wheel”
“I favour a mixture of strontium and copper. The energy emitted when the electrons return to their lower energy state is highly agreeable.”
Now THAT’S good chat.





